Change Notification
Introduction:
The medical device industry is constantly evolving therefore change is inevitable, and how medical device industries manage change will definitely impact their business including internal processes and the products that they design, develop, manufacture, and distribute into the market. Change management process in these establishments will not only impact their work but also patients’ lives. When the needs for change occur, establishments have to make sure all changes made are managed properly and everything is accounted for in the quality management system and the resulting documentation and records.
Change Notification is the process to notify the Authority on the changes made to the registered medical device within the validity period of medical device registration. Types of changes are included but not limited to the following;
i. Change in manufacturing facility, process and quality management system (QMS)
ii. Changes in design or specifications of a registered medical device
iii.Changes to materials in a general / IVD medical device
iv.Changes to labelling of medical devices
v. Changes to registered medical devices registration information
Changes in medical devices may take place from time to time as part of their life-cycle. Any change to a registered medical device is linked to the principles of safety and performance and the ability of the regulatory framework to manage the risk of the medical devices.
Before making any decision whether a changed medical device can continue to be placed in the market, the Authority will determine whether evidence of safety and performance have been appropriately collected and reviewed based on the notification made by the registration holder.
For any anticipated change to a medical device, a manufacturer must consider the impact of the change on the patient, practitioner and/or user of the medical device, and the impact of the change on the specifications of the medical device, and decide whether the change is expected to impact the safety and performance of the medical device.
- Guidance Documents:
- Application for Change Notification:
c. Category of Change
Remark: If there is any dispute information between the Guidance Document of Change Notification (MDA/GD/0020) and Medcast 2.0+ system, it is advisable to oblique with the Guidance Document requirement.
d. Frequently Ask Questions (FAQs)
e. Any inquiries, please email to: registration@mda.gov.my
f. Contact No. relevant Officer
- Norfazila Bt Zulkifli
Email: norfazila@mda.gov.my
Phone: 03-8230 0399
2. Norul Hasmida Bt Ibrahim
Email: norulhasmida@mda.gov.my
Phone: 03-8230 0235
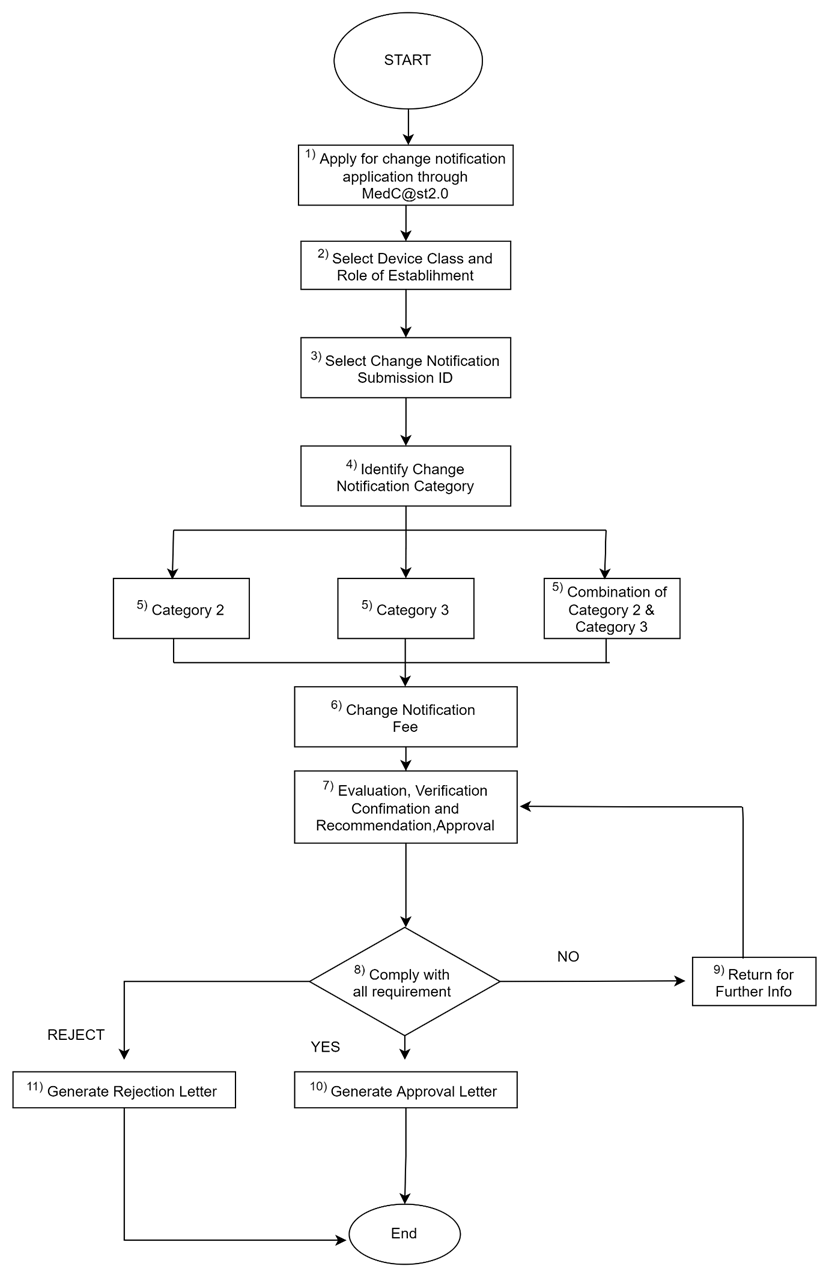
h. Process flow:
Flowchart for Single Application
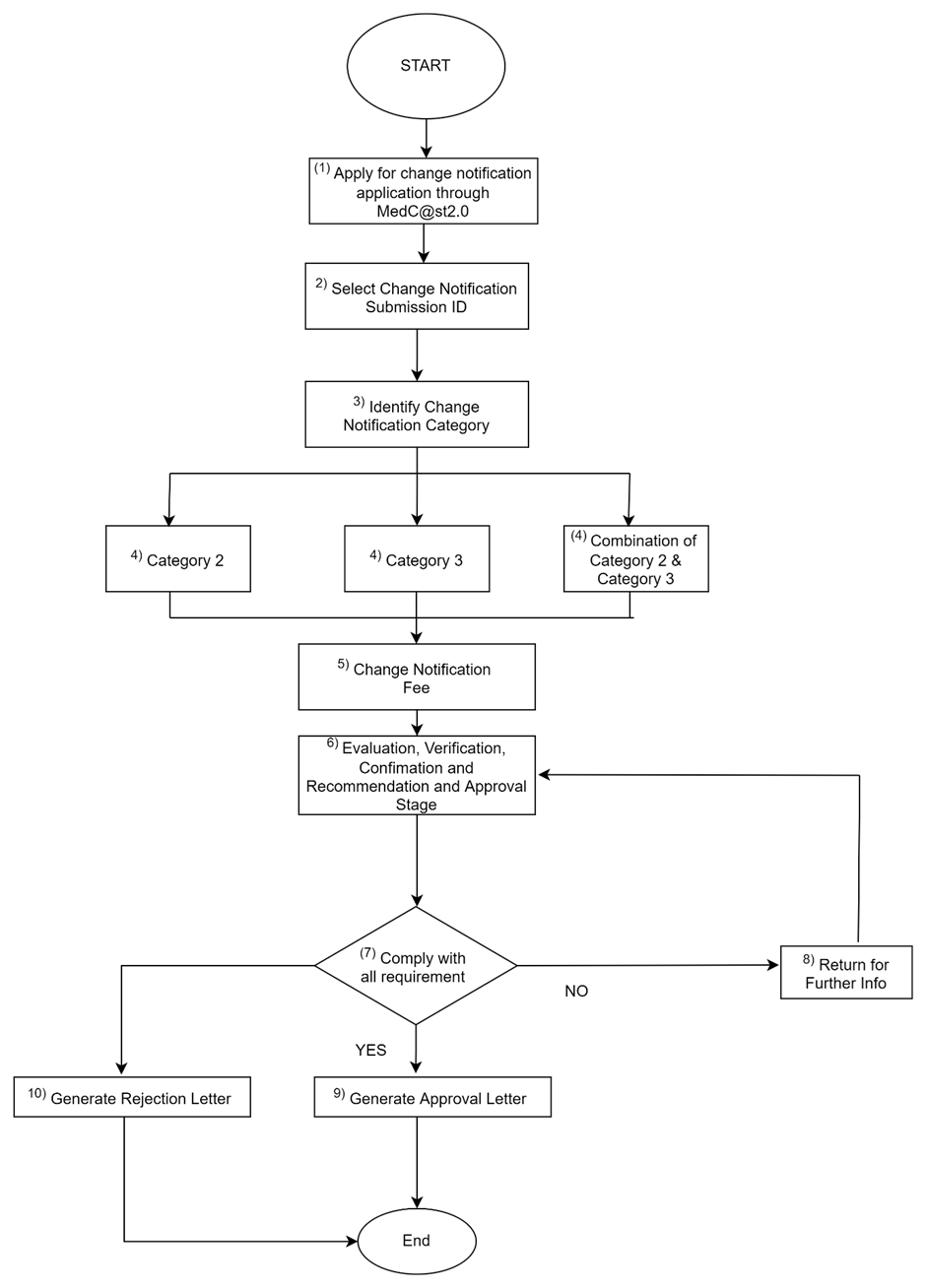
Flowchart for Multiple Application