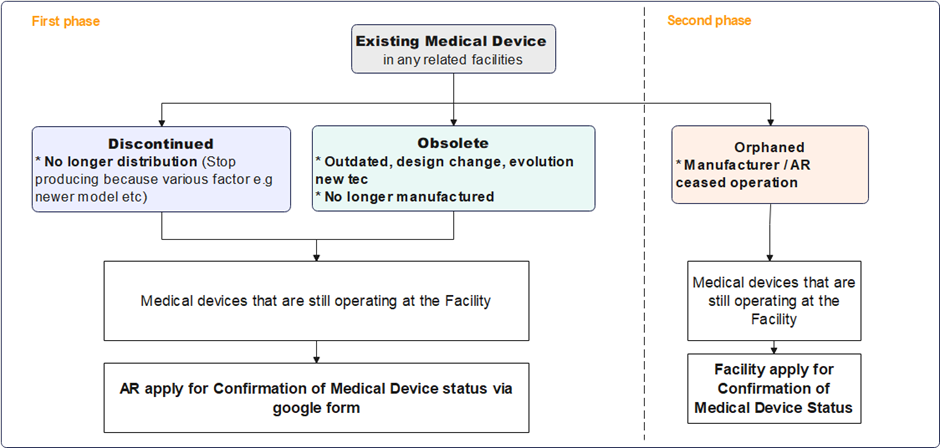
Obsolete, Discontinued & Orphaned Medical Device
Full Implementation of Notification for Orphaned, Obsolete, and Discontinued Medical Devices
Update :
The Medical Device Authority (MDA) would like to provide an update on the implementation timeline for the Notification of Orphaned, Obsolete, and Discontinued (OOD) Medical Devices. This initiative, originally set to launch in full by November 2024, will now be introduced in two phases to ensure comprehensive guidance and smooth implementation:
- Phase 1: Discontinued and Obsolete (OD) Medical Devices
The guidance document for OD medical devices is in the final stages of completion, and the application for exemption process for these categories will commence following its release.
- Phase 2: Orphaned Medical Devices
The development of a separate guidance document specifically for orphaned medical devices is underway. The application for exemption process for orphaned devices will begin upon completion of this additional guidance document.
Suggestion Framework (Updated version, will be notified):
Any inquiries, please email to : ood.md@mda.gov.my
Updated: 30th Oct 2024






