Special Access Medical Device
Definition : Medical device for the use of medical practitioners in emergency situations or in the event that conventional medical treatment has failed, is unavailable or unsuitable.
** Essential Requirement: Adhere to this prior to submission -(click here)-
1. Notification form : Medcast
2. How to create Medcast Account : Medcast – Notification Creation
3. Administrative Charge : RM 300
4. Post handling : Disposal of medical device for special access Form
5. Guidance Documents : Special Access – Notification – General Requirements. "Document under revision"
6. Any inquiries, Please email to sa.cm@mda.gov.my
7. Contact No. relevant Officer
-
- Puan Haidar Nadirah Bt Hawalig +603-8230 0250
- Puan Nur Maizura Bt Zarmani +603-8230 0339
- Puan Aidahwaty Bt Ariffin +603-8230 0341
- En. Aznil (Printing Officer) +603-8230 0247
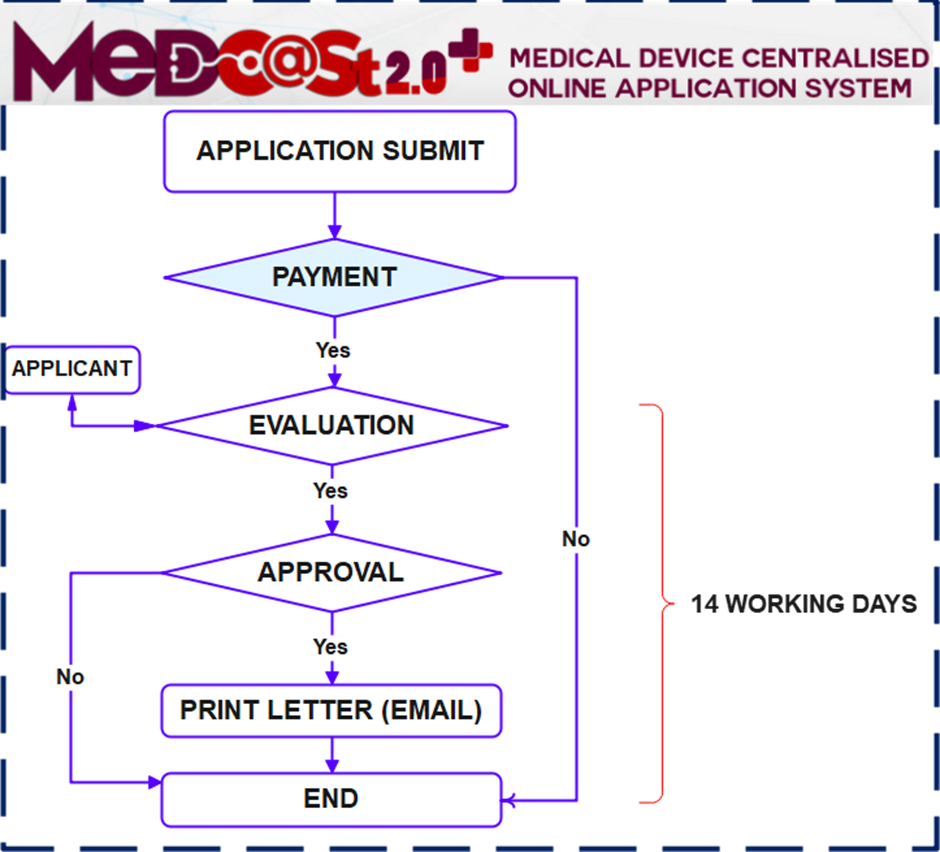
Process flow:
Importance Notice :
-
Please be advised that Special Access route is strictly for medical devices required in certain situations especially when no suitable registered alternative is available. Applications that do not meet these criteria or lack proper justification will be rejected.
-
To qualify for Special Access Notification, all applicants must possess a valid establishment license, specifically as an Authorized Representative and Importer.
-
Effective from September 1, 2023, strict adherence to the essential requirement is mandatory. Applications that do not align with these stipulations might face rejection. In the event of an application being returned, the designated time frame for resubmission is limited to 7 working days.
Updated: 8th October 2025






