ANNOUNCEMENT: PILOT SURVEY FOR UNIQUE DEVICE IDENTIFICATION (UDI) IN MALAYSIA
Dear Medical Device Sector Stakeholders,
ANNOUNCEMENT: PILOT SURVEY FOR UNIQUE DEVICE IDENTIFICATION (UDI) IN MALAYSIA
Greetings from the Medical Device Authority.
On the 7th of June, MDA launched the first phase of the pilot survey for Unique Device Identification (UDI) implementation in Malaysia. The survey will be carried out in two phases and will consist of the following establishments and timelines:
Phase 1: Class D Medical Device Establishment - 7th of June 2024 until 31st of July 2024
Phase 2: Class B Medical Device Establishment - 15th of August until 15th of October 2024
The purpose of this survey is to find out the readiness of the medical device establishments in Malaysia to provide all the information required regarding UDI for their registered products.
UDI is a unique numeric or alphanumeric code that consists of a Device Identifier (DI) and Production Identifier (PI) that serves to adequately identify medical devices sold in Malaysia from manufacturing through distribution to patient use.
The results obtained from the survey will help the Authority to decide on the implementation timeline and new updates in our MeDC@St 3.0+.
The timeline of the UDI implementation is shown in the diagram below.
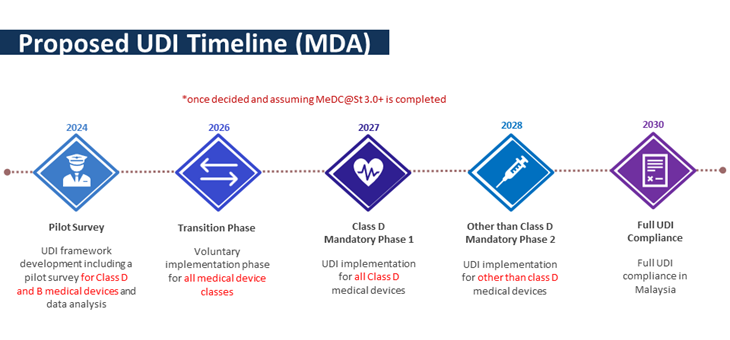
Therefore, to ensure reliable data collection, MDA urges the mentioned establishment to give full cooperation in the pilot survey.
Thank you.
Policy and Strategic Planning Division
Medical Device Authority
10 July 2024






