ANNOUNCEMENT ON MDA CONSULTANCY SERVICES
MDA offers regulatory consulting that can support stakeholders who are involve in regulatory submission for medical devices in Malaysia, to comply with medical device regulatory requirements under Medical Device Act 2012 (Act 737). The regulatory consulting services include:
Online consultancy maybe requested for applicant who isn’t physically able to meet MDA’s consultancy team. Consultancy *fee imposed is based on type of consultancy services selected.
*Note: Please refer to Consultancy Request Form (Package) and Consultancy Request Form (Non-Package)
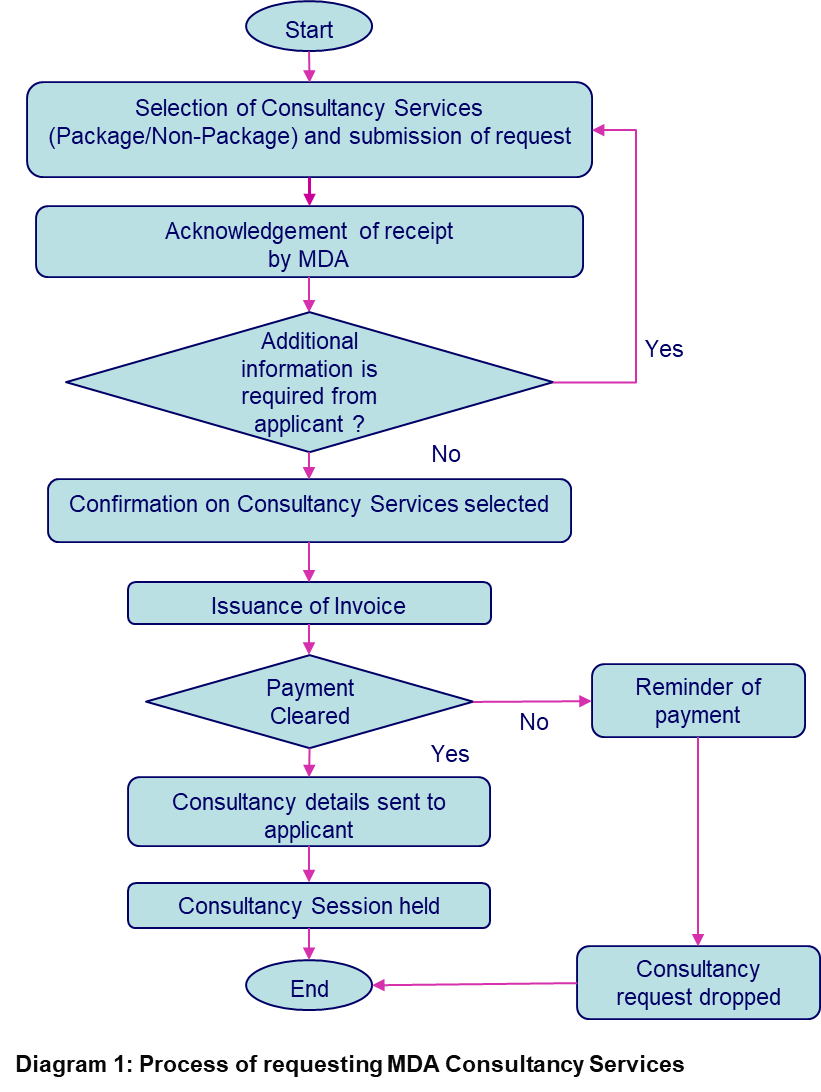
The following diagram describes the step-by-step process of requesting our consultancy services:

If you want to know more about our consultancy services, send an email to consultation@mda.gov.my.
Updated: 2/8/2023