Archived Speeches by the CEO of MDA
Sedang Dikemaskini
Media Coverage and Newspaper Clippings
Sedang Dikemaskini
Malaysian Leadership
Dimuatnaik oleh : Unit Komunikasi Korporat
Disediakan oleh : Unit Komunikasi Korporat
Dikemaskini pada : 19 Mei 2025
Former CEO of MDA


Disediakan oleh : Unit Komunikasi Korporat
Dikemaskini pada : 19 Mei 2025
EXPORT CERTIFICATE APPLICATION PROCESS
Introduction
The Export Certificate (EC) is a certificate issued by MDA as a service to Malaysia exporters to export the Export Only Medical Device from Malaysia to a foreign country.
An Export Only Medical Device Exemption Letter issued by MDA shall be first obtained prior to the Export Certificate application.
Note: For more information on Export Only Medical Device Exemption Letter, please Click Here
Application procedure
The applicant who needs Export Certificate in order to comply with importing country requirements is required to submit an application via Google Form together with all supporting documents listed below:
|
No |
Supporting Documents |
|
1 |
|
|
2 |
Export Only Medical Device Exemption Letter issued by MDA |
|
3 |
|
|
4 |
Medical device label (shall be provided based on importing country information as stated in the application form) |
|
5 |
Table 1: Supporting documents for export certificate
The EC will be issued by MDA upon satisfactory review of all the supporting documents provided as required and subject to payment of the fees.
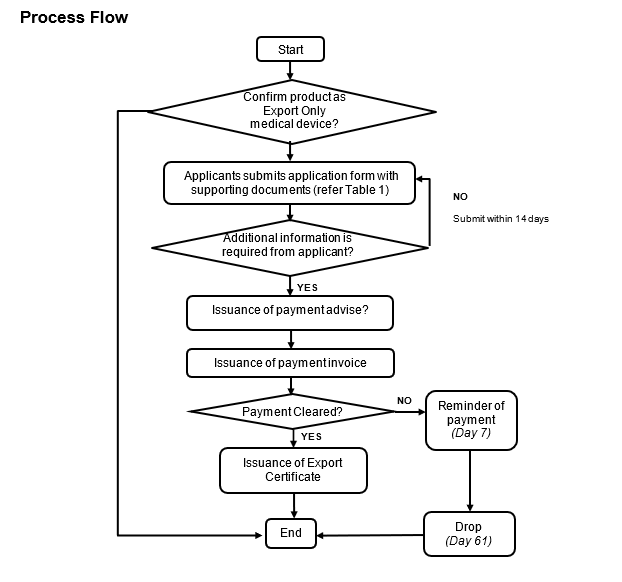
The turn-around time is 14 working days with complete documentation.
Fees
Below are the applicable fees
i) Application fee: RM 250.00
ii) Export certificate fee. The fee for EC is as follows:
|
No |
Validity |
Fee (RM) |
|
1 |
2 years |
100 |
|
2 |
5 years |
300 |
|
- Calculation is based per number of countries requested. - Certificate is inclusive of 1 attachment. Additional pages will be charged RM5.00 per page. Each attachment will list up to 25 medical devices. - Certificate will be issued based on per country. |
||
|
Export Certificate Calculation Fees |
||
|
i. |
1 Country 100 medical devices 1 copy of certificate Validity 2 years |
25 medical devices per attachment thus require 4 attachments. (complimentary 1 attachment). RM 250 (application fee) + RM 100 (2 years validity) + RM15 (Attachment) = RM 365 |
|
ii. |
1 Country 100 medical devices 2 copies of certificate Validity 2 years |
25 medical devices per attachment thus require 4 attachments. (complimentary 1 attachment). RM 250 (application fee) + RM 100 (2 years validity) + RM15 (Attachment) = RM 365 for 1 EC For 2 copies: RM 250 (application fee) + (RM 120 x 2) = RM 485 |
|
iii. |
2 Countries 1 medical device 1 copies of certificate Validity 5 years |
RM 250 (application fee) + [RM 300 (Validity 5 years) x 1 (Attachment) x 1 (copy) x 2 (countries)] = RM 850 |
The payment shall be made online via BayarNow system. Click on BayarNow for more information.
For any inquiries, please email to exportonly.ec@mda.gov.my
The contact number of the relevant officers:
|
1. |
Mdm. Norhafizah |
03-8230 0208 |
|
2. |
Mr. Fezri |
03-8230 0395 |
|
3. |
Mr. Shahrezza |
03-8230 0392 |
Updated: 25 October 2023






