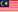Obsolete, Discontinued & Orphaned Medical Device
Full Implementation of Notification for Orphaned, Obsolete, and Discontinued Medical Devices
Update :
The Medical Device Authority (MDA) would like to provide an update on the implementation timeline for the Notification of Orphaned, Obsolete, and Discontinued (OOD) Medical Devices. This initiative, originally set to launch in full by November 2024, will now be introduced in two phases to ensure comprehensive guidance and smooth implementation:
- Phase 1: Discontinued and Obsolete (OD) Medical Devices
The guidance document for OD medical devices is in the final stages of completion, and the application for exemption process for these categories will commence following its release.
- Phase 2: Orphaned Medical Devices
The development of a separate guidance document specifically for orphaned medical devices is underway. The application for exemption process for orphaned devices will begin upon completion of this additional guidance document.
Suggestion Framework (Updated version, will be notified):
Any inquiries, please email to : ood.md@mda.gov.my
Updated: 30th Oct 2024
List of Device Studies (Device Study Tracker)
![]()
Please click link to view list of device studies tracker as below:
https://docs.google.com/spreadsheets/d/1BR9xYVYDdvv2mi0QO7pp1r4NIeyHn18Qv3mf0AhylSI/edit?usp=sharing
Updated: 29th March 2023
Special Access Medical Device
Definition : Medical device for the use of medical practitioners in emergency situations or in the event that conventional medical treatment has failed, is unavailable or unsuitable.
** Essential Requirement: Adhere to this prior to submission -(click here)-
1. Notification form : Medcast
2. How to create Medcast Account : Medcast – Notification Creation
3. Administrative Charge : RM 300
4. Post handling : Disposal of medical device for special access Form
5. Guidance Documents : Special Access – Notification – General Requirements. "Document under revision"
6. Any inquiries, Please email to sa.cm@mda.gov.my
7. Contact No. relevant Officer
-
- Puan Haidar Nadirah Bt Hawalig +603-8230 0250
- Puan Nur Maizura Bt Zarmani +603-8230 0339
- Puan Aidahwaty Bt Ariffin +603-8230 0341
- En. Aznil (Printing Officer) +603-8230 0247
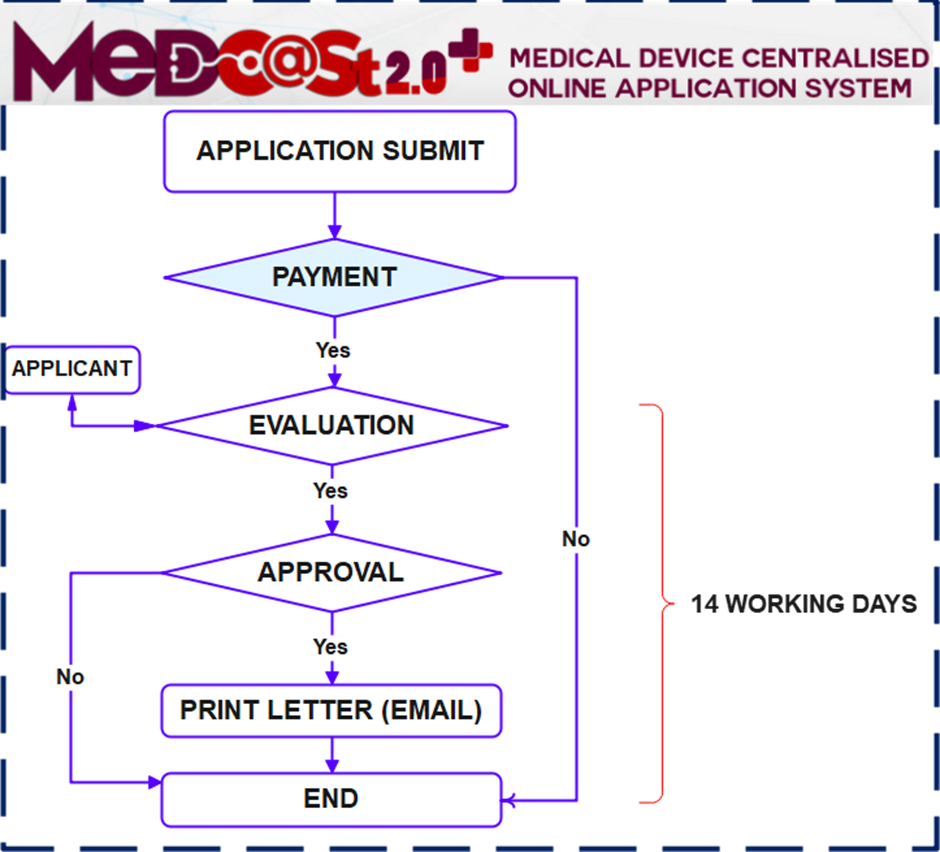
Process flow:
Importance Notice :
-
Please be advised that Special Access route is strictly for medical devices required in certain situations especially when no suitable registered alternative is available. Applications that do not meet these criteria or lack proper justification will be rejected.
-
To qualify for Special Access Notification, all applicants must possess a valid establishment license, specifically as an Authorized Representative and Importer.
-
Effective from September 1, 2023, strict adherence to the essential requirement is mandatory. Applications that do not align with these stipulations might face rejection. In the event of an application being returned, the designated time frame for resubmission is limited to 7 working days.
Updated: 8th October 2025
Custom-made Medical Device
|
Announcement: Clarification on the regulation of Dental Products The Medical Device Authority (MDA) wishes to clarify that dental products, including but not limited to crowns and aligners, are medical devices that fall under the regulatory requirements of the Medical Device Act 2012 [Act 737]. Accordingly, all local companies including laboratories involved in the manufacturing of such dental products must ensure that these devices are registered with the Authority to comply with Act 737. In cases where the dental products meet the criteria for custom-made medical devices, manufacturers may submit an exemption application directly to the Authority. This exemption is applicable only when the specific requirements of a custom-made device are fulfilled. This announcement serves to reinforce the importance of regulatory compliance and to support the safe and effective use of dental products in Malaysia. Date of published: 11-Nov-2024 |
Custom made Criteria :
1. Designed and manufactured according to a written prescription from a qualified medical practitioner for the sole use of a particular patient.
2. Does not fall into the category of mass-produced medical devices that require adaptation for specific professional user requirements.
** Medcast form requirement: - Crucial to adhere (click here) -
How to apply?
A. Notification form : Medcast
B. How to create Medcast Account : Medcast – Notification Creation
C. Administrative Charge : RM 300
D. Guidance Documents : NOTIFICATION OF CUSTOM-MADE (MDA/GD/0064)
E. Any inquiries, please email to sa.cm@mda.gov.my
F. Contact No. relevant Officer:
- Puan Haidar Nadirah Bt Hawalig +603-8230 0250
- Puan Nur Maizura Bt Zarmani +603-8230 0339
- En. Mohamad Aznil bin Ahmad Azmi (Printing Officer) +603-8230 0247
Process flow:

Importance Notice :
- Kindly note that commencing 1 December 2023, all submissions for Custom Made Medical Device Notifications are required to be made through the MeDC@St 2.0 online platform. The application processing period is 14 working days upon receipt of a complete application.
- The Custom-Made Notification System is activated using earlier version of Medcast 2.0. Users may face system bugs, but rest assured, it won't impact your application's submission. Applicants are advised to refer the Medcast Form Requirement to prevent any potential issues. If you encounter unresolved issues, please reach out to the MDA officer for assistance.
Updated: 8th October 2025