COMPLIANCE AUDIT
Latar Belakang
Cawangan audit pematuhan telah ditubuhkan pada tahun 2012 dan diletakkan di bawah Bahagian Dasar, Kod dan Standard, Pihak Berkuasa Peranti Perubatan (PBPP). Terdapat 3 orang pegawai ditugaskan di bawah cawangan ini bagi menjalankan kerja-kerja audit pematuhan ke atas establismen dan Badan Penilaian Pematuhan (CAB).
Fungsi dan objektif
Fungsi dan objektif cawangan audit pematuhan adalah bagi memastikan establismen dan CAB sentiasa mematuhi keperluan regulatori dan apa-apa keperluan yang telah ditetapkan oleh PBPP. Berikut merupakan jenis audit yang dijalankan.
Jenis Audit yang dijalankan:
- Audit ke atas CAB:
- Audit Pendaftaran – Audit yang dijalankan ke atas CAB bagi tujuan pendaftaran dengan PBPP.
- Audit Survelan – Audit yang dijalankan bagi tujuan pemantauan ke atas CAB yang berdaftar dengan PBPP
iii. Witness/Observe Audit – Audit Penyaksian ke atas auditor dari CAB yang berdaftar
- Audit ke atas establishmen
- Audit Survelan – Audit yang dijalankan bagi tujuan pemantauan terhadap establismen yang berlesen dengan PBPP. Audit ini merangkumi skop ISO 13485 bagi audit / pemeriksaan ke atas pengeluar, dan skop Good Distribution Practice of Medical Device (GDPMD) bagi audit / pemeriksaan ke atas wakil diberi kuasa (AR), pengedar dan pengimport.
Policy, International Affairs and Industry Facilitation (DASAR)
POLICY, INTERNATIONAL AFFAIRS AND INDUSTRY FACILITATION (DASAR)
- Develop, plan, coordinate and implement;-
i - policy development and legal documents such as rules and regulations relating to the control of medical devices under the Medical Devices Act 2012 (Act 737),
ii - the development of guidance documents, codes and standards relating to the control of medical devices under the Medical Device Act 2012 (Act 737) and its rules in more detail,
iii - development of GLP recognized laboratory
- Develop, plan, coordinate and implement activities pertaining to multilateral cooperation and relationship between agencies within and outside the country,
- Develop, plan, coordinate and implement activities pertaining to industrial assistance
- Develop, plan, coordinate and implement compliance monitoring activities against legal requirements under Act 737 through audits and inspection.
i- Policy & Industrial Assistance Branch consisting of 3 units namely International Policy & International Unit, Code & Standard Development Unit and Industrial Assistance Unit,
ii- Compliance Audit Branch conducting CAB audit activities, product and premise quality management and regulatory audit.
Compliance Branch
COMPLIANCE BRANCH
Background
The Compliance Branch formerly known as the Audit Branch was established in 2012. It is operated under the Policy, Code and Standards Division, Medical Device Authority (MDA).
Functions
To ensure that the establishment and Conformity Assessment Body (CAB) continuously comply with the Act 737, Medical Device Regulation, condition of license / registration and other directives set by the MDA through the inspections
Types of inspections conducted as per below;
Inspection on CAB:
- Pre-registration Inspection – Conducted on a company who wish to register as a CAB in Malaysia to ensure they are comply to Act 737 and Fourth Schedule of MDR 2012 prior to registration
- Surveillance Inspection – Conducted on registered CAB from time to time to ensure CAB continuously complies to condition of registration, CAB’s QMS is implemented according to Malaysia Regulatory Requirements and information submitted in the online system (MeDC@St) is up to date
- Witness / Observe Inspection – Conducted on auditors of registered CAB from time to time to verify the CAB’s ability to assign auditors based on registered scope / code, adequacy of the auditor’s auditing practices against Malaysia Regulatory Requirements and auditor ability to generate reliable audit reports.
Inspection on Establishment
- Surveillance Inspection – Conducted from time to time on licensed establishment (manufacturer / authorised representative/ importer / distributor) to ensure the establishment complies to conditions of license, QMS is established and implemented according to Malaysia Regulatory Requirements and information submitted in online system (MeDC@St) is up to date
Terjemahan kandungan di atas
PEMATUHAN PEMANTAUAN
Latar Belakang
Cawangan Pematuhan yang dahulunya dikenali sebagai Cawangan Audit telah ditubuhkan pada tahun 2012. Ia dikendalikan dibawah Bahagian Dasar, Kod dan Standard, Pihak Berkuasa Peranti Perubatan (PBPP)
Fungsi
Memastikan Establismen dan Badan Penilaian Pematuhan (CAB) mematuhi keperluan Akta 737, peraturan peralatan perubatan, syarat lesen/pendaftaran dan arahan lain yang ditetapkan oleh MDA secara berterusan melalui pemeriksaan
Jenis pemeriksaan yang dijalankan seperti di bawah;
Pemeriksaan ke atas CAB
- Pemeriksaan Pra-Pendaftaran – dijalankan ke atas syarikat yang ingin mendaftar sebagai CAB di Malaysia bagi memastikan mereka mematuhi Akta 737 dan jadual keempat MDR 2012 sebelum pendaftaran
- Pemeriksaan Surveilan – Dijalankan di atas CAB berdaftar dari semasa ke semasa bagi memastikan CAB mematuhi syarat pendaftaran, sistem pengurusan kualiti yang dilaksanakan mengikut keperluan regulatori Malaysia secara berterusan. Selain itu, maklumat yang dikemukakan dalam sistem dalam talian (MeDC@St) betul dan dikemaskini
- Pemeriksaan Penyaksian – Dijalankan ke atas Juruaudit CAB berdaftar dari semasa ke semasa untuk mengesahkan keupayaan CAB untuk menugaskan Juruaudit berdasarkan skop/kod yang berdaftar, kompetensi Juruaudit terhadap keperluan regulatori Malaysia dan keupayaan juruaudit untuk mengeluarkan laporan audit yang konsisten dan boleh dipercayai
Pemeriksaan ke atas Establismen
- Pemeriksaan Surveilan– dijalankan dari semasa ke semasa ke atas establismen berlesen (pengilang/wakil diberi kuasa/pengimport/pengedar) untuk memastikan establismen mematuhi syarat-syarat lesen, sistem pengurusan kualiti telah diwujudkan dan dilaksanakan mengikut keperluan regulatori dan maklumat yang dikemukakan dalam sistem atas talian (MeDC@St) adalah betul dan dikemaskini
Industrial Support
The carried out activities are;
Certificate of free sales (CFS) and Manufacturing certificate
To provides a service to medical devices industries with handles application of Certificate of Free Sale (CFS) and Manufacturing Certificate (MC). This certification is intended to help medical device industries engaged in the exportation.
Involvement with the ministry/department/others agency
Participated in a series of discussions with the relevant agencies to discuss new matters and resolve issues related to medical devices in Malaysia.
Greetings and Welcome
Greetings and welcome to the official website of the Medical Device Authority (MDA).
 The MDA website contains a wealth of information about medical device and its regulatory control for the benefit of the consumers, patients, medical device industry - namely manufacturers, authorised representatives of foreign manufacturers, importers, distributors, conformity assessment bodies, medical professionals and public as a whole.
The MDA website contains a wealth of information about medical device and its regulatory control for the benefit of the consumers, patients, medical device industry - namely manufacturers, authorised representatives of foreign manufacturers, importers, distributors, conformity assessment bodies, medical professionals and public as a whole.MDA is a statutory body under the Ministry of Health Malaysia which was established under the Medical Device Authority Act 2012 (Act 738) to control, regulate medical device, its industry and activities as well as to enforce medical device law under Medical Device Act 2012 (Act 737). Prior to the enactment of Act 738, the Medical Device Control Division (MDCD) of Ministry of Health Malaysia was entrusted to develop the medical device regulatory program, including the drafting of medical device law. Subsequent to the gazettement of Act 738, MDA was formed in May 2012 (and MDCD ceased to operate). Officers and staffs of the then MDCD were transferred to MDA in June 2012.
It has come a long way since the time the regulatory program was first proposed in 2005 until the date when Act 737 finally comes into effect on 30 June 2013 and then followed by the effective date of Medical Device Regulations 2012 on 1 July 2013. The regulatory reform started with the determination of policies, study on regulatory practices in established countries, engagement with stakeholders and coming up with an appropriate regulatory model for Malaysia. The regulatory model was developed based on best regulatory practices and is consistent with the global harmonised model. It has two main aims, namely to protect public health and safety and to facilitate medical device trade and industry.
With the effective dates of Act 737 and Medical Device Regulations 2012, the medical device industry has now progressed from unregulated environment to a regulated one and the industry players must observe all the applicable regulatory requirements stipulated by the Authority under Act 737. However, as provided in Section 80, Act 737 offers a transition period of two years and one year respectively for the industry to submit applications for medical device registration and establishment licensing before the law is fully enforced.
" BERILTIZAM PENUHI HASRAT "
Any comment or feedback please e-mail to : mdb@mda.gov.my
MeDC@St
MeDC@St is a web-based Online Application System for Establishment Licensing, Medical Device Registration. It is a centralized system where only one account needs to be created by an applicant to apply for Establishment Licensing and Medical Device Registration.
- Introduction on Medical Device, please click here!
- For more information, please click here!
- MeDC@St FAQ, please click here!
- TO APPLY for Establishment Licence, Medical Device Registration, In-Vitro Diagnostic (IVD) Medical Device Registration, please Create an Account OR Login here!
WHAT IS MeDC@St?
(1) MeDC@St is a platform for the industry players to submit their applications for registration of medical devices and licensing of establishments under Act. It is a fully web-based online application system which enables submissions to be made from anywhere in the world, which also provides features that enable access by multiple users.
HOW TO CREATE A MeDC@St ACCOUNT?
(2) Figure 2 shows the steps to be taken by an applicant to create a MeDC@St account for the purpose of making application for registration of medical device under Act 737.

(3) Three main steps are involved in MeDC@St account creation
Step 1:
(i) An applicant needs to complete MeDC@St Account Creation Form and must provide information required in MeDC@St Account Creation Form which includes
- Business registration,
- Establishment name, and
- A valid email address.
(ii) User name and password are also required for security purposes. An applicant must provide and reconfirm the password. If the form is completed, applicant must click <create account> button to proceed to next step.
Step 2:
(iii) Upon creation of a MeDC@St account, a validation email will be sent to the email address provided in the form to activate the account. Applicant needs to log in to his/her email account to validate the email address provided in the MeDC@St Account Creation Form.
Note: A validation email may be received in the SPAM or TRASH folder because of filtering by receiving email server. If the validation email is not received in the inbox, please check in the SPAM or Trash folder.
Step 3:
(iv) After the email is validated, applicant must login to the system by providing the User Name and Password given in the MeDC@St Account Creation Form during account creation.
HOW TO APPLY
 How to Apply for Establishment Licence Under Medical Device Act 2012 (Act 737), please click here!
How to Apply for Establishment Licence Under Medical Device Act 2012 (Act 737), please click here!
 Guidelines on Medical Device Registration Under Act 737, please click here!
Guidelines on Medical Device Registration Under Act 737, please click here! 
 How to Apply for In-Vitro Diagnostic (IVD) Medical Device Registration under Medical Device Act 2012 (Act 737), please click here!
How to Apply for In-Vitro Diagnostic (IVD) Medical Device Registration under Medical Device Act 2012 (Act 737), please click here!
INFORMATION ON FEE
A. As prescribe in the Fifth Schedule of the Medical Device Regulation; the application fee is imposed as follows;
1. Application Fee for Medical Device Registration
|
Medical Device |
Fee Payable (RM) |
|
A Class A medical device |
100 |
|
A Class B medical device |
250 |
|
A Class C medical device |
500 |
|
A Class D medical device |
750 |
2. Registration Fee
|
Medical Device |
Fee Payable (RM) |
|
A Class A medical device |
- |
|
A Class B medical device |
1,000 |
|
A Class C medical device |
2,000 |
|
A Class D medical device |
3,000 |
|
A medical device that contains a medicinal product |
5,000 |
|
Application Type |
Fee Payable (RM) |
|
New application |
|
|
i- Manufacturer |
250 |
|
ii- Authorised Representative |
250 |
|
iii- Distributor |
250 |
|
iv- Importer |
250 |
|
Renewal application |
|
|
v- Manufacturer |
200 |
|
vi- Authorised Representative |
200 |
|
vii- Distributor |
200 |
|
viii- Importer |
200 |
|
Application Type |
Fee Payable (RM) |
|
New Licence |
|
|
i- Manufacturer |
4,000 |
|
ii- Authorised Representative |
4,000 |
|
iii- Distributor |
2,000 |
|
iv- Importer |
2,000 |
|
Renewal Licence |
|
|
v- Manufacturer |
2,000 |
|
vi- Authorised Representative |
2,000 |
|
vii- Distributor |
1,000 |
|
viii- Importer |
1,000 |
PAYMENT INSTRUCTION
i. All fees shall be paid 30 days after notifications on the payment advice are notified in the MeDC@St Account. Applications will be dropped from the system if the payment is not received within the specified time.
ii. All fees shall be paid through bank draft. CASH is NOT accepted. We will not be responsible for the cash sent or brought to our office.
iii. The bank draft must be made payable to "KUMPULAN WANG PIHAK BERKUASA PERANTI PERUBATAN" and sent to:
KETUA EKSEKUTIF
Level 6, Prima 9, Prima Avenue II,
Block 3547, Persiaran APEC,
63000 Cyberjaya, Selangor, MALAYSIA
U/P: UNIT KHIDMAT PENGURUSAN
iv. Information on Application Submission ID and Phone No. of the Contact Person must be written at the back of the bank draft but not in the table (see example below).
v. The PAYMENT ADVICE must be printed and attached together with bank drafts to indicate the details of payment.
vi. Payment for application fees and registration fees of different class of medical devices CANNOT be combined in one bank draft. It is adviceable to split the payment in different bank drafts. The fees of maximum of 5 submissions can be combined together in a bank draft.
vii. The acceptance of the bank draft is subject to the evaluation by our Finance Unit and the recipient bank.
viii. The bank draft can be returned back to the applicant if payment information is inaccurate.
ix. A receipt of payment will be issued once the bank draft is accepted. The receipt must be kept as proof of payment.
x. Please be ensured that the bank draft is still valid when it reaches MDA as bank drafts have validity period i.e. 2 months).
xi. All fees are non-refundable regardless the decision made by the Authority pertaining to the application made under the Medical Device Act 2012 (Act 737).
EXAMPLE OF BANK DRAFT PREPARATION

Organization Chart
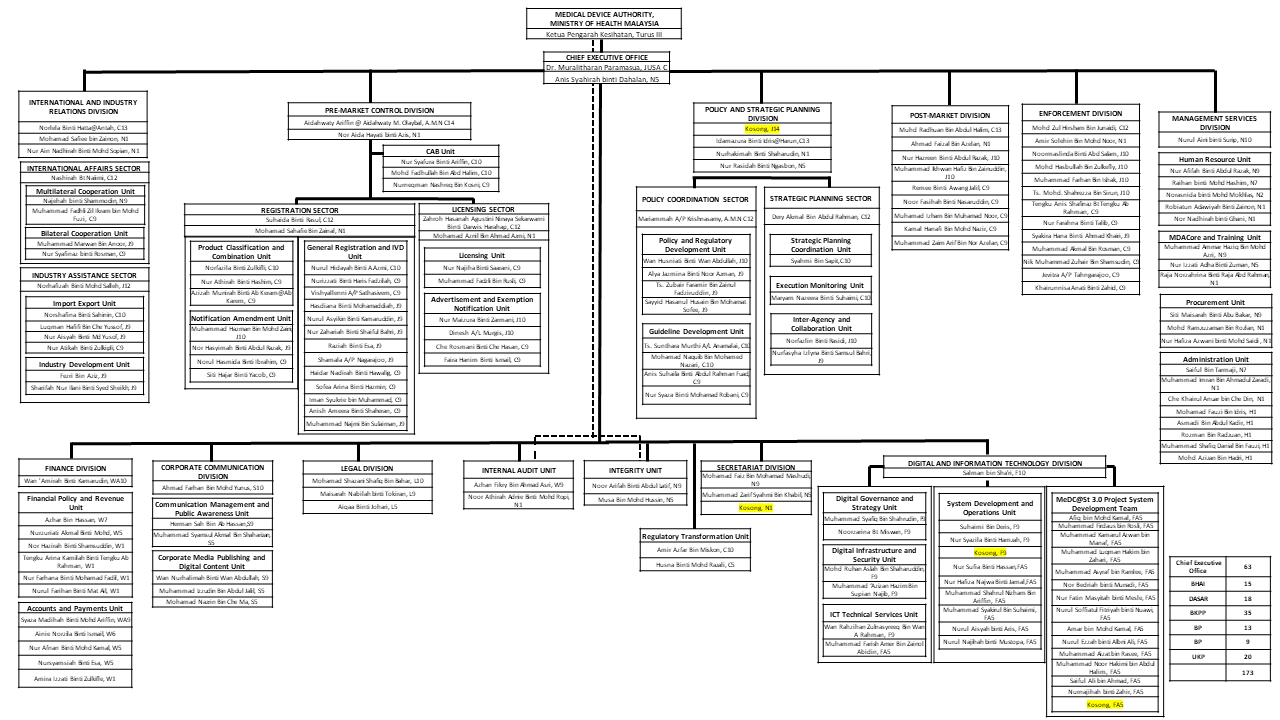
Updated on: 27 / 10 / 2025
Prepared by: Administrative Division
Uploaded by: Corporate Communication Division